How Is the Electronegativity Trend Related to the First Ionization
Exceptions to this trend is observed for alkaline earth metals group 2 and nitrogen. First ionization energy of lithium.

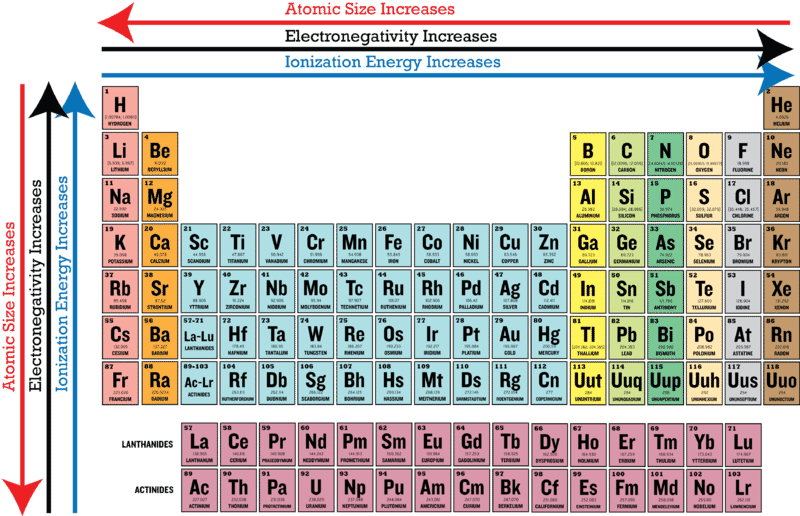
Easy To Use Chart Of Periodic Table Trends Teaching Chemistry Chemistry Classroom Science Chemistry
Please give reasons for compounds like silver sulfate or silver chloride to be insoluble that relate to lattice energy versus energy of hydration.

. The trend is shown below. When we go down in a group in the periodic table electronegativity decreases. Since going from right to left on the periodic table the atomic radius increases and the ionization energy increases from left to right in the periods and up the groups.
This was just a brief layout of the trends in electronegativity of an element in the periodic table. Periodic Table and Trend of Ionization Energies. Here fluorine has the highest electronegativity 40.
As described above ionization energies are dependent upon the atomic radius. It is one of the most reactive elements in the periodic table therefore usually only found in compoundsIt tends to oxidize in air very rapidly thus accounting for its rapid reaction with oxygen when freshly exposed to air. Potassium K is an alkali metal placed under sodium and over rubidium and is the first element of period 4.
The trend in electronegativity can be seen by the graph given below for group 7.
Periodic Trends In Electronegativity Ck 12 Foundation

Periodic Trends Chemwiki Electron Affinity Ionization Energy Chemistry Textbook

Periodic Trends Electronegativity Ionization Energy And Atomic Radius Teaching Chemistry Ionization Energy Chemistry Lessons

How Is Electronegativity Related To Ionization Energy And Electron Affinity Quora
No comments for "How Is the Electronegativity Trend Related to the First Ionization"
Post a Comment